Colligative Properties Freezing Point Depression Lab Answers
Other physical pharmacy topics important to drug formulation are discussed in the chapters that follow which describe. Two of the new experiments have been added to Chapter 11.
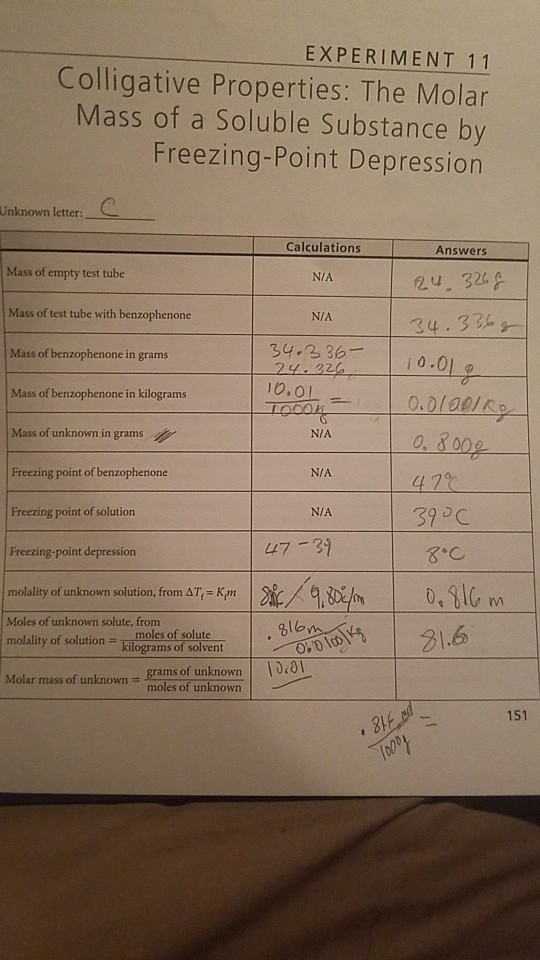
Solved Experiment 11 Colligative Properties The Molar Mass Chegg Com
Thermochemistry kinetics and colligative properties- 1967 Amount of Substance-Source Wikipedia 2013-09 Please note that the content of this book primarily consists of articles available from Wikipedia or other free sources online.

. FREEZING POINT DEPRESSION INTRODUCTION LABORATORY SIMULATION Lab Data Freerine-coint of Diwata C Verify your calculation - X GRAPH Sell 020 CHEA Solution 1 Solution 2 Solution 3 HOW KCI КСІ КСІ 7455 7455 7455 1745 1722 472 1730 190 480 100 213 354 Freezing point of. Place it in beaker so temperature is maintained 4. Record temperature every 30 seconds.
Colligative Properties - CHEM 1252L - UNC Charlotte - StuDocu The colligative properties include vapor pressure lowering boiling point elevation freezing point depression and osmotic pressure. Colligative Properties Freezing Point Depression Lab Answers Keywords. Colligative Properties Lab Freezing Point Depression Boiling Point ElevationThe physical properties of solutions that depend on the number of dissolved solute particles and not their specific type are known as colligative properties.
Freezing point depression occurs when a solute is added to a solvent producing a solution having lower freezing point temperature than the pure solvent. These include freezing point depression osmotic pressure and boiling point elevation. Using Freezing Point Depression Colligative Properties COLLIGATIVE PROPERTIES Pre-Lab - NYB Chemistry of Solutions AP Chem.
This new framework allows the evaluation of the full range of benefits and shortcomings of substitutes and examination of. ACCOMPANY YOUR SPARE TIME WHEN BEING AT HOMEColligative Properties Freezing Point Depression Lab June 26th 2018 - Document Read Online Colligative Properties Freezing Point Depression Lab Answers Colligative Properties Freezing Point Depression Lab Answers In this site is not the same as a answer Experiment 12 Freezing Point of Solutions. D T Km where D T is the change in freezing or boiling point K is a solvent-specific constant and m is the solutions molality.
Chemical Engineering questions and answers. These include freezing point depression osmotic pressure and boiling point elevation. The colligative-property law can be expressed using the equation.
Colligative properties vapor pressure lowering freezing point depression u0026 boiling point eleDepression in Freezing Point - Solution and Colligative Properties - Chemistry Class 12 Determining Molar Mass of. Once naphthalene has melted remove tube and place model in a beaker. Put naphthalene in tub remove stopper and place in a 600 ml beaker of water.
Colligative Properties Freezing Point Depression Lab Answers Author. Construct a model using a stopper thermometer tube and wire. ΔT f T f solution - T f pure solvent K f m 1 where ΔT f is the freezing temperature K f is the molal freezing-point depression constant a property of a given solvent and m is the molality of solute in the solution.
In terms of and a non-colligative properties why is this difference relevant. And freezing point depression The Colligative Properties Boiling-Point Elevation and Freezing-Point Depression Freezing point depression - Ice Cream Lab 2014 CBSE Class 12 Chemistry Solutions 7 Colligative Properties. The freezing point depression can be determined by subtracting the temperature of the solution by the temperature.
A Discuss the difference between an electrolyte electrolyte. The vapor pressure is the escaping tendency of solvent molecules. Since the freezing point is lowered the observed freezing point of this solution will be 806oc - 88oc 718oc Since the molal freezing-point-depression constant is known it is possible to obtain the molar mass of a solute by measuring the freezing point of a solu-tion and the weight of both the solute and solvent.
Osmotic Pressure A demonstration of Colligative Properties Ice Cream and Freezing Point Depression. Colligative properties freezing point depression lab answers Created Date. Get Free Colligative Properties Freezing Point Depression Lab Answers how modern information sources such as computational modeling can supplement traditional toxicology data in the assessment process.
Design experiments to answer a research question about the influence adding a solute has to the solvents physical properties. Download Ebook Colligative Properties Freezing Point Depression Lab Answers concepts and others set the framework for the subsequent chapters that describe physicochemical properties and process related to the fate of the drug. Access Free Pre Ap Freezing Point Depression Lab Answers Freezing Point Depression Pre-Lab - Alyssa Chang Mrs Ross.
Freezing point and the concentration of a solution is given by the following. In this case a solution will begin to boil at a higher temperature than its corresponding pure solvent from the following equation. The freezing point depression of Solution 1 is -44 C and the freezing point depression of Solution 2 is -77 C.
Colligative Properties Freezing Point Depression Lab Answers Understanding Chemistry. Pre-labs and questions were revised and several experiments were added or changed. A corollary colligative property to freezing point depression is boiling point elevation.
Chemistry questions and answers. Experiment are boiling point elevation and freezing point depression. ColligativeProperties Lab Freezing Point Depression Boiling Point Elevation.
Solution Manual for Physical Chemistry - DOKUMENPUB as colligative properties. ΔT b K b m Here ΔT b is the increase between the pure solvents and the solutions boiling points K b is the. The physical properties of solutions that depend on the number of dissolved solute particles and not their specific type are known as colligative properties.
Freezing point and boiling point. Define the term colligative property and list the four common colligative properties of solutions. What influence does adding more solute to a solvent hae on the freezing point and boiling point of the resultant solution compared to the pure solvent.
These include freezing point depression osmotic pressure and boiling.

Colligative Properties Lab Freezing Point Depression
Freezing Point Depression With Antifreeze Stephanie S Wonderful World Of Ap Chem Labs

Colligative Properties Lab Freezing Point Depression Boiling
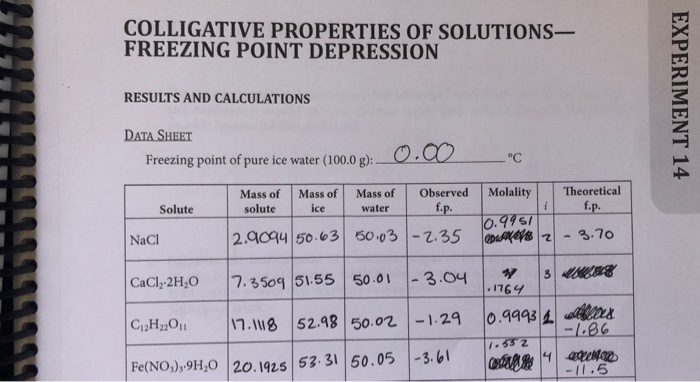
Solved Colligative Properties Of Solutions Freezing Point Chegg Com
Comments
Post a Comment